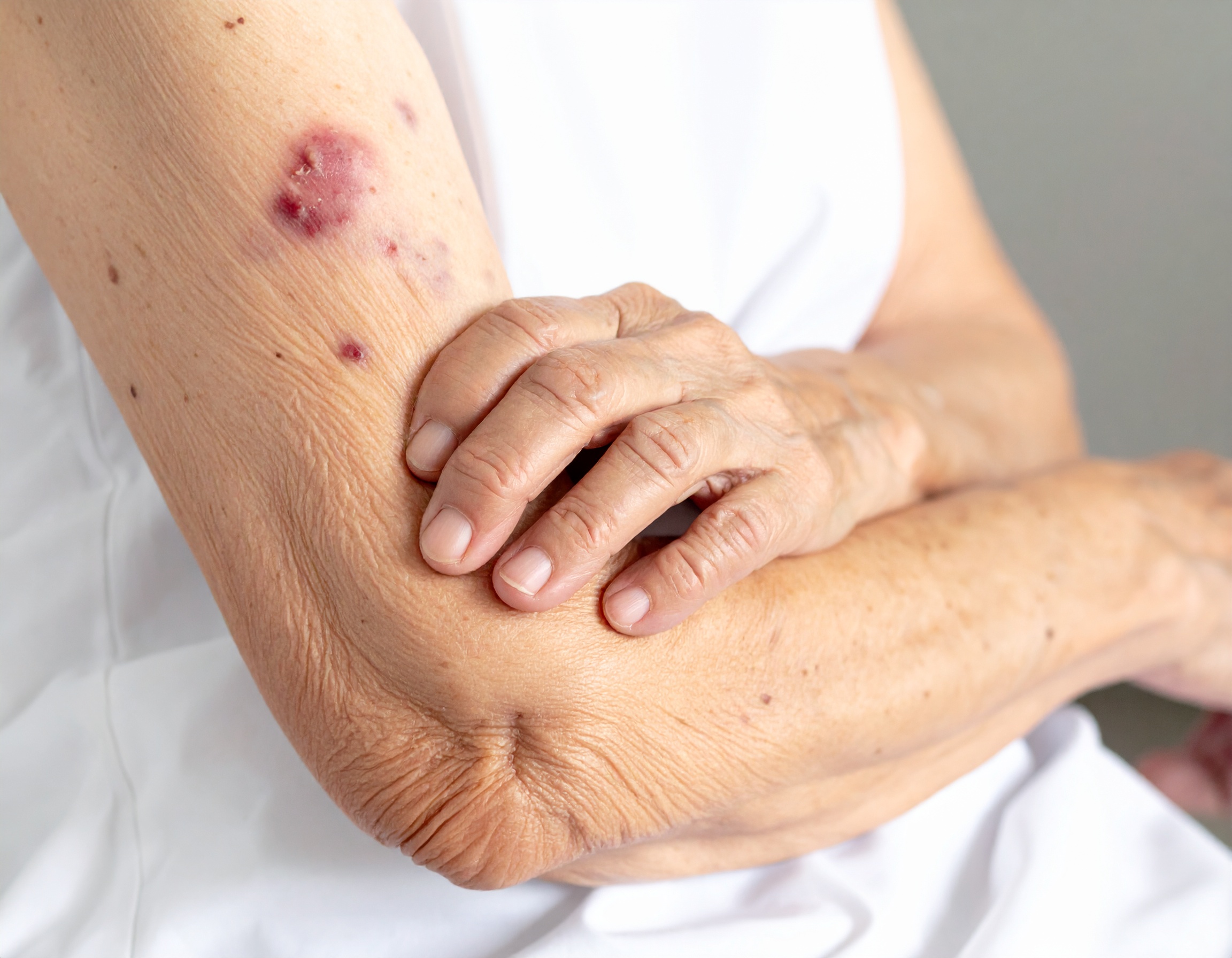
In this case scenario, we will look at the importance of making an apixaban (podcast) dose adjustment based upon clinical factors. LS is an 83-year-old female with a history of atrial fibrillation, hypertension, and osteoarthritis. She’s currently prescribed Eliquis (apixaban) 5 mg twice daily for stroke prevention and diltiazem 180 mg daily for rate control. Over the last month, nursing staff have reported frequent bruising on her arms and legs, even from light contact. Her most recent labs show a hemoglobin of 10.8 g/dL (down from 12.0 g/dL), serum creatinine 1.6 mg/dL, and weight of 56 kg (123 lbs).
When reviewing her medications, the pharmacist identifies that LS meets at least two of the three dose reduction criteria for apixaban: age ≥80 years, body weight ≤60 kg, and serum creatinine ≥1.5 mg/dL (excellent board exam nugget). Because at least two are met, the recommended dose should be reduced to 2.5 mg twice daily for stroke prevention in atrial fibrillation.
The presence of diltiazem further complicates the situation. Diltiazem is a moderate CYP3A4 and P-glycoprotein inhibitor, which can increase apixaban concentrations and raise bleeding risk. The combination of her advanced age, reduced kidney function, weight, and concurrent diltiazem likely explains her bruising and drop in hemoglobin.
The pharmacist recommends reducing apixaban to 2.5 mg twice daily, monitoring hemoglobin and renal function, and reassessing the need for diltiazem. If rate control can be maintained with a beta-blocker, that may further reduce bleeding risk. Education is provided to nursing and the prescriber to monitor closely for any additional signs of bleeding or anemia.
Always review apixaban dose criteria in older adults. This case represents an appropriate time to make an apixaban dose adjustment. This is especially true if there are signs of adverse effects. In this case the bruising and slight drop in hemoglobin are potentially problematic. If two of the following are present—age ≥80, serum creatinine ≥1.5 mg/dL, or body weight ≤60 kg—reduce the dose to 2.5 mg BID. Concomitant CYP3A4/P-gp inhibitors like diltiazem, verapamil, or amiodarone can further elevate exposure and increase bleeding risk. Monitoring and medication reconciliation are critical to prevent adverse outcomes.


0 Comments