Parkinson’s disease (PD) has long presented challenges in medication delivery. A staple in more advanced disease control, carbidopa-levodopa requires multiple daily dosing with supratherapeutic levels leading to dyskinesias and subtherapeutic concentrations leading to troublesome “off” time. Sinemet (carbidopa-levodopa), Sinemet Controlled-Release (CR), and their respective generic equivalents have been used in PD for decades, but leave a lot to be desired in optimizing medication delivery. Rytary (carbidopa-levodopa ER) is a new drug formulation approved in 2015 with a slightly alternative pharmacokinetic profile.
In clinical trials, Rytary showed benefit compared to Sinemet IR in decreased daily “off” time (1 hr) and increased “on” time without dyskinesia (0.9 hr). Additionally, Rytary was dosed on average 3.6 times daily compared to 5 times daily for Sinemet IR. Direct efficacy comparisons of Rytary to Sinemet CR are lacking, but pharmacokinetic profiles and clinical practice standards speak to the possible benefits of Rytary. Sinemet CR has unpredictable absorption and delayed time to clinical effect (1-3 hr) so IR tablets are often taken at the same time for quicker onset. Rytary may also provide benefits from decreased concentration variability at the steady-state and increased duration of action compared to CR.
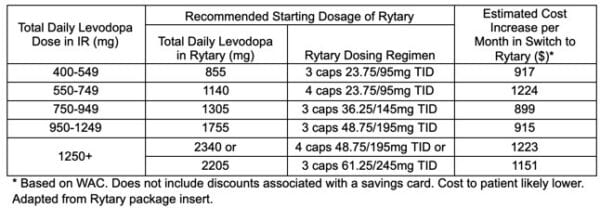
Rytary’s altered pharmacokinetics compared to traditional carbidopa-levodopa combinations is due to a formulation primarily comprised of both IR and ER beads. IR beads allow for drug absorption similar to Sinemet IR with an initial Tmax of 1 hour while ER beads maintain levels at >50% of Cmax for 4-5 hours. The delayed absorption with ER beads reduces the total amount of levodopa that is available to the body, thus daily amounts of levodopa in Rytary are considerably higher than intake from IR and CR formulations (~2-fold and ~1.5-fold, respectively). See the table below for recommended starting dosing when converting from a stable IR regimen.
The benefits of Rytary do come at a big cost. As a brand-name product that is often not well covered by insurance, patients are likely going to be paying more for the convenience of fewer daily doses and better dopaminergic control. The table below highlights estimated cost increases per month for Rytary compared to IR products. Rytary may be an option for patients with PD that are still experiencing frequent “off” episodes or troublesome dyskinesias from their current Sinemet IR/CR regimen.
In clinical practice, I’ve personally only seen this medication used a couple of times in patients who have tried and failed numerous combinations of other less expensive levodopa-carbidopa combinations. The most common adverse effects of Rytary are going to be similar to those of its less expensive counterparts (i.e. nausea, hallucinations, orthostasis, etc.). While we can never have too many options to choose from, the expense will often limit the use of this levodopa-carbidopa formulation.
Article written by Evan Odean in collaboration with Eric Christianson, PharmD, BCGP, BCPS
- 30 medication mistakes PDF
- 18+ Page Drug Interaction PDF
- 10 Commandments of Polypharmacy Webinar based on my experiences in clinical practice
Study Materials and Resources For Healthcare Professionals and Students – Amazon Books
Sources:
Product Information: Rytary, levodopa an carbidopa capsule, extended release. [package insert]. Amneal Pharmaceuticals LLC, 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6c1f7cd4-de56-45c1-a734 5e77b4aeb6f7. Accessed 16 February, 2021.
Hauser Robert A, Hsu Ann, Kell Sherron, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson’s disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurology 2013; 12: 346-356.
Mittur Aravind, Gupta Suneel, and Modi Nishit B. Pharmacokinetics of Rytary, An Extended Release Capsule Formulation of Carbidopa-Levodopa. Clinical Pharmacokinetics 2017; 56 (9): 999-1014.
Carbidopa-levodopa. Micromedex Solutions Red Book. Micromedex Solutions [online]. Updated 12 February, 2021. IBM. Accessed 16 February, 2021.


0 Comments