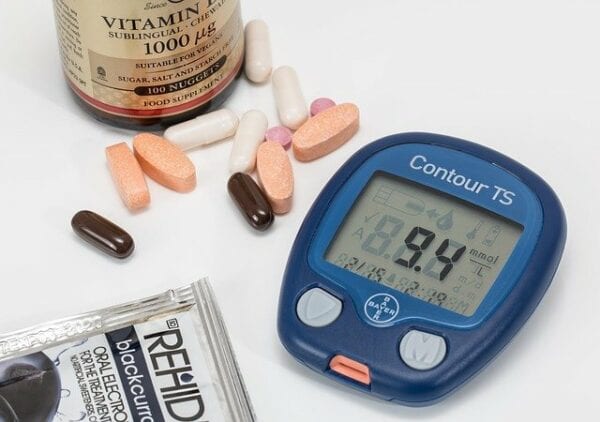
Technology continues to impact disease state management in remarkable ways. Monitoring of patients with diabetes is no exception. Both the American Diabetes Association and American Association of Clinical Endocrinologists/American College of Endocrinology make light of this in their most recent guidelines for usage of continuous glucose monitoring (CGM) devices. Broadening language now recommends consideration of these technologies in certain patients with type 1 or type 2 diabetes, whether they require treatment with insulin or not. We want to outline a little more detail about CGM in general and will cover some common medications that interfere with continuous glucose monitoring.
CGM measures glucose concentrations in the interstitial fluid of subcutaneous tissue, which is a briefly delayed, but representative estimate of blood glucose concentrations. The data gathered from these instruments can help fine-tune glucose control in ways not previously possible, resulting in reduced frequency of hypoglycemia events and lowered hemoglobin A1c. Glucose levels from an inserted probe are transmitted to an external device for painless monitoring and trending levels can even provide alerts to users to prevent highs or lows. As an additional benefit, most devices can now be worn for 10-14+ days which greatly reduces finger sticks in patients with diabetes, especially for devices that do not require frequent calibration.
Although results are likely to be more dramatic in patients with type 1 diabetes or those on intensive insulin therapy, use of CGM has shown benefit and may be considered in patients with type 2 diabetes who are not meeting glycemic targets, have recurrent hypoglycemia, or hypoglycemia unawareness, even if not using insulin.
Common commercially available products such as Dexcom, FreeStyle Libre, Medtronic Guardian, and Eversense are gaining use with the release of proven benefits and better insurance coverage. Most private insurance will now cover CGM for patients with type 1 diabetes as well as patients with type 2 diabetes using multiple doses of insulin daily. As an alternative, temporary continuous glucose monitors can be placed by healthcare personnel to record 1-2 weeks of data with follow-up analyses and interpretation of results reported back to a patient. These types of products/services are called professional (Pro) CGM and can be billed for by physicians and other healthcare clinicians based on individual state laws.
One potential drawback of some CGM models is that substances such as acetaminophen, ascorbic acid (vitamin C), hydroxyurea, tetracycline antibiotics, and salicylic acid (aspirin) may falsely elevate glucose readings. This can result in the failed notification of a patient who is hyper- or hypoglycemic. Newer products aim to eliminate these issues, but it is worth checking the individual device before dispensing or educating a new user. The table below lists major CGM products and medications that interfere with continuous glucose monitoring.
| APAP | Salicylic Acid | Ascorbic Acid | Hydroxyurea | Tetracyclines | |
| Dexcom G6 | Nonea | None | Yes (↑↑)e | ||
| Medtronic Guardian 3 | Yes (↑)b | ||||
| Senseonics Eversense | None | None | Yes (↓) | ||
| Abbott FreeStyle Libre 2 | None | Yes (↓)c | Yes (↑)d | ||
| Medtronic iPro2 | Yes (↑)b | ||||
| Dexcom G6 Pro | Nonea | Yes (↑↑) | |||
| Abbott FreeStyle Libre Pro | None | Yes (↓)c | Yes (↑)d |
(b) The degree of inaccuracy varies based on the individual and the amount of acetaminophen present
(c) May mildly reduce glucose readings
(d) Doses of more than 500mg daily increase readings. One study suggests doses of 1-3g daily increase readings 9.3-20 mg/dL.
(e) Glucose readings averaged 76 mg/dL above actual levels after ingestion and remained high for 6 hours post-dose
* Blank cells have not been well studied
In addition to the table above, there is also some information regarding potential interference with lisinopril, albuterol, atenolol, and red wine.
The takeaway message is to always test with a conventional glucose meter if symptoms do not match a CGM reading and to be aware of how certain devices may be impaired by common medications. In addition, if you use these devices, become familiar with the strengths and weaknesses of each device pertaining to medications that interfere with continuous glucose monitoring.
In my practice, I have seen these technologies being used in patients with both type 1 and type 2 diabetes. Have you seen CGM benefit your patients? Have you seen any issues with medications that interfere with CGM? Comment below!
The article was written by Evan Odean in collaboration with Eric Christianson, PharmD, BCGP, BCPS
- 30 medication mistakes PDF
- 18+ Page Drug Interaction PDF
- 10 Commandments of Polypharmacy Webinar based on my experiences in clinical practice
Sources:
Bakris George, Blonde Lawrence, Boulton Andrew JM, et al. Standards of Medical Care in Diabetes. Diabetes Care 2021; 44: S1-S232.
Product Information: FreeStyle Libre 14 day, Flash Glucose Monitoring System. [package insert]. Abbott Diabetes Care, Inc, 2018. https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160030S017C.pdf Accessed 8 February, 2021.
American Association of Clinical Endocrinologists. Continuous Glucose Monitoring (CGM), 2020. https://pro.aace.com/pdfs/diabetes/AACE-DRC-CGM-Slides.pdf. Accessed 8 February, 2021.
FreeStyle Libre Pro. Professional Continuous Glucose Monitoring. Billing and Reimbursement Guide, 2016. Abbott Diabetes Care, Inc. https://provider.myfreestyle.com/pdf/ADC-02243v2.pdf. Accessed 8 February, 2021.
Centers for Medicare & Medicaid Service. Physician Fee Schedule. Updated 20 January, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search/overview. Accessed 8 February, 2021.
Tellez Siobhan E, Hornung Lindsey N, Courter Joshua D, et al. Inaccurate Glucose Sensor Values After Hydroxyurea Administration. Diabetes Technology & Therapeutics, 2020; Ahead of print.
Lorenz Carrie, Sandoval Wendolyn, and Mark Mortellaro. Interference Assessment of Various Endogenous and Exogenous Substances on the Performance of the Eversense Long-Term Implantable Continuous Glucose Monitoring System. Diabetes Technol Ther., 2018; 20(5) 344 352.
User Guides for G6, Guardian, Libre, Eversense.


Thanks Eric, really helpful and interesting. Do we know the science behind why the above drugs specifically cause this?
Many CGMs work by detecting voltage change as glucose oxidase catalyzes the oxidation of glucose. Some small molecules appear to interfere with this detection method. See this excerpt from the Basu et al. (https://pubmed.ncbi.nlm.nih.gov/26784129/).
“Because glucose detection with CGM probes relies on an electrochemical reaction, certain pharmaceuticals that are small molecules could interfere with CGM glucose detection especially if (a) their volume of distribution is large enough for the compound to reach the ISF and (b) the oxidation voltage is smaller than the working voltage used in CGM devices. Acetaminophen is a representative example of a commonly used medication that possesses such properties that increases its likelihood of interference with CGM glucose, which makes CGM interference testing even more of a priority for improving overall care of patients using CGM devices.”
The article goes on to describe methods that could attenuate these effects, which appears to be what more recent models of the Dexcom (and less successfully, Medtronic) products have implemented.
“CGM manufacturers have two basic options for reducing the susceptibility of CGM sensors to electrochemical interferences. One method for reducing the susceptibility to pharmacologic interferences is to change the working voltage of the sensor…The other method for reducing the susceptibility to pharmacologic interferences is to apply a permselective membrane to the sensor surface that inhibits the diffusion of potential pharmacologic interferences into the interior of the sensor. This is presumably the approach taken more recently by Dexcom and Medtronic with their latest-generation CGM systems.”
The Eversense product uses a completely different mechanism which is based in fluorescence technology. The Lorenz article cited above does a good job of explaining how Eversense is different (https://pubmed.ncbi.nlm.nih.gov/29600877/).
That’s fascinating, thanks Evan. Very much appreciated.
I have been using the Freestyle Libre 2 i am on my second sensor I have noticed that when I take an asprin 500 mg my sensor readings drop low to around 67-54 ML about two hours after I take it and will rise back up to normal around three hours later is this normal and would like to know if there is and group that helps out with cost of sensors i was able to get my first two free because my health care Humana does not cover
I started taking ciproflaxin for A UT1. Will that interfere with my Dexcom G6?