Obesity is a major risk factor for many comorbidities (hypertension, dyslipidemia, osteoarthritis, sleep apnea, heart disease, type 2 diabetes) and increases all-cause and cardiovascular mortality. With over 40% of Americans qualifying as obese (body mass index ≥30 kg/m2) in 2017-18 it is no surprise that weight loss remains a hot ticket item today. Medications for weight loss (or chronic weight management) should be offered to overweight and obese patients who are motivated to lose weight as adjunctive therapy to lifestyle modifications when diet and exercise are not sufficient.
Lifestyle and behavioral adjustments are always first-line treatments for weight loss. Typically, a daily caloric deficit (energy intake – energy output) of at least 500 kcal is recommended in addition to a minimum of 150 weekly minutes of moderate-intensity aerobic exercise. Approved weight loss medications were all studied in addition to these lifestyle modifications so it is important to stress the continuation of diet and exercise plans when starting pharmacologic treatment. Prior to initiating therapy, conduct a thorough medication review to determine medications that may be contributing to weight gain. Many drugs used to prevent pregnancy or treat diabetes, psychosis, epilepsy, or depression may have this adverse effect. Patients with a BMI of ≥27 kg/m2 and at least 1 obesity-related comorbid condition (such as those listed above) or a BMI ≥30 kg/m2 may be offered medication treatment for chronic weight management. To obtain this indication, therapies should demonstrate in large trials clinically significant weight loss (5% or more of total body weight) versus placebo that is maintained at 1 year or longer. Currently, there are 4 FDA-approved medications in this class.
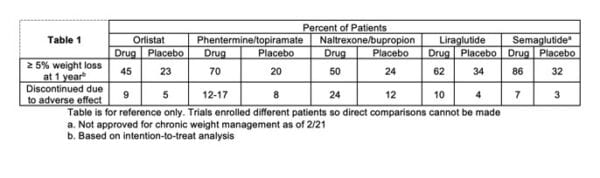
Orlistat was the first medication approved for long-term weight loss. It is available by prescription as Xenical or OTC as Alli. Prescription doses of 120mg TID with high-fat meals show benefit in weight loss. This medication works by inhibiting pancreatic and gastric lipases thereby reducing the absorption of dietary fats. Based on this mechanism there are multiple side effects (oily spotting, flatulence with discharge, fecal incontinence, etc) and drug interactions (levothyroxine, cyclosporine, warfarin, amiodarone, anti-epileptic drugs, etc). The most common reason that patients have difficulty adhering to this treatment is the GI side effects. Patients should take a multivitamin containing fat-soluble vitamins at least 2 hours post-dose once daily to avoid a deficiency. Orlistat is contraindicated in pregnancy or patients with cholestasis or chronic malabsorption. See Table 1 below for treatment versus placebo comparisons of clinically significant weight loss and adverse effects leading to discontinuation of treatment. The average weight loss compared to placebo was 3% after 1 year. This medication is likely the safest option for most patients, with little systemic drug absorption. It may also be the most inexpensive, especially if OTC is used off-label at Rx doses. Standard Medicare plans do not cover any weight-loss drugs, but some commercial plans may cover Xenical in tier 3.
Phentermine/topiramate is sold under the brand name Qsymia. Phentermine is a stimulant medication that increases norepinephrine and satiety while topiramate’s effect in weight loss may be due to lipogenesis. To avoid insomnia, dosing is in the morning and starts at 3.75mg phentermine/23mg topiramate ER for 2 weeks. Dosing is doubled at 2 weeks and can reach a max of 15/92mg daily. The drug should be stopped if 5% weight loss is not achieved after 12 weeks on the max dose. There is a REMS program in place for this medication due to fetal harm in pregnancy. It is contraindicated in pregnancy, glaucoma, hyperthyroidism, and within 14 days of an MAOI and caution is emphasized with sleep disorders, CV disease, and severe liver impairment. Side effects include paresthesia, irritability, metallic taste, insomnia, and hypokalemia (when used with a loop or thiazide diuretics). See Table 1 below for treatment versus placebo comparisons of clinically significant weight loss and adverse effects leading to discontinuation of treatment. Qsymia demonstrates robust weight loss (~10% on average) and is a good option for people without uncontrolled hypertension or heart disease. It is covered by many commercial plans in lower tiers with PA or step-therapy so it may be one of the cheaper options as well.
Naltrexone/bupropion is a combination of an opioid antagonist and antidepressant which targets craving and reward centers in the brain to help with weight loss. It is marketed as the brand name Contrave. Dosing is titrated weekly for the first month of therapy. There is a warning for suicidal thoughts and behaviors since this medication includes an antidepressant. It should not be used in patients with uncontrolled hypertension, chronic opioid use, seizure disorder, or within 14 days of an MAOI. It could be the weight loss drug of choice for people with concomitant depression, those that describe food cravings, smokers that want to quit, or people who want to reduce alcohol consumption. Nausea, vomiting, constipation, and headache are the most common adverse effects and led to 24% of people discontinuing trials versus 12% of placebo. Fifty percent achieved clinically significant weight loss at 1 year compared to 24% of placebo-treated participants with a mean weight loss of around 8%. Some commercial plans do cover Contrave in tier 3, but it remains one of the more expensive options. Contrary to orlistat, Contrave should not be taken with a high-fat meal due to increased drug exposure. Contrave is supplied as 8mg naltrexone/90mg bupropion ER tablets. Patients take 1 tablet in the morning for the first week and then add 1 tablet weekly until a max dose of 2 tablets twice daily is reached after 4 weeks.
Liraglutide is a GLP-1 receptor agonist first approved for the treatment of type 2 diabetes. This medication is approved as the brand name Victoza for T2DM (max dose of 1.8mg daily) but is used at higher doses in weight loss as Saxenda (titrated to 3mg daily). It is a daily subcutaneous injection. Weight loss is achieved through decreased caloric intake as this medication decreases gastric emptying and targets brain areas responsible for satiety. Adverse effects are similar to other GLP-1 receptor agonists and include nausea, constipation, and diarrhea. See Table 1 below for treatment versus placebo comparisons of clinically significant weight loss and adverse effects leading to discontinuation of treatment. Mean weight loss is around 6% with Saxenda. Saxenda is contraindicated in pregnancy and those with certain types of thyroid cancers. It is likely the most expensive weight loss therapy but may be a good option for patients with diabetes or those taking psychiatric medications who aren’t ideal candidates for the options listed above.
As discussed in a recent blog post, semaglutide will likely be the next agent approved for chronic weight management in the coming months. This drug demonstrated massive weight losses with 86% of patients reaching a 5% or more total weight loss at 1 year compared to 32% in the placebo arm. See table 1 below for relative comparisons between weight loss medications.
It is critical to note that all trials discussed here enrolled different patients based on slightly different parameters. Thus, direct comparisons between outcomes should not be made. There are no head-to-head trials between approved medications for weight loss (though a trial of semaglutide versus liraglutide is ongoing). Additionally, no trials have shown a benefit in regard to mortality.
The most common drug prescribed for long-term weight loss is not approved for this indication. Phentermine as monotherapy is often used as it is inexpensive and relatively safe. Long-term (greater than 3 months) efficacy data is lacking with phentermine. However, continued therapy is reasonable if a patient is seeing a benefit, not experiencing adverse effects, and has no psychiatric disease history or predisposition to dependence.
Which medications do you see most often used for weight loss?
- 30 medication mistakes PDF
- 18+ Page Drug Interaction PDF
- 10 Commandments of Polypharmacy Webinar based on my experiences in clinical practice
Article was written by Evan Odean in collaboration with Eric Christianson, PharmD, BCGP, BCPS
Study Materials and Resources For Healthcare Professionals and Students – Amazon Books
References:
Centers for Disease Control and Prevention. Adult Obesity Facts. Updated 11 February, 2021. https://www.cdc.gov/obesity/data/adult.html. Accessed 26 February, 2021.
Jensen Michael D, Ryan Donna H, Apovian Caroline M, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2014: 129; S102-S138.
Apovian Caroline M, Aronne Louis J, Bessesen Daniel H, et al. Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015: 100 (2); 342 362.
Product Information: Xenical, orlistat capsule. [package insert]. H2-Pharma LLC, 11/2020.
Product Information: Qsymia, phentermine and topiramate capsule, extended release. [package insert]. Vivus, Inc., 10/2020.
Product Information: Contrave Extended-Release, naltrexone hydrochloride and bupropion hydrochloride tablet, extended release. [package insert]. Nalpropion Pharmaceuticals LLC, 10/2020.
Product Information: Saxenda, liraglutide injection, solution. [package insert]. Novo Nordisk, 12/2020.
Wilding John PH, Batterham Rachel L, Calanna Salvatore, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. NEJM 2021. DOI: 10.1056/NEJMoa2032183
Image courtesy of https://totalshape.com/



0 Comments
Trackbacks/Pingbacks